Buy Xeloda : Capecitabine 500 Mg Tablets Online
$165.33
Brand Name : Xeloda
Composition : Capecitabine
Manufactured by : Roche Holding AG
Strength : 500 mg
Form : Tablets
Packing : Pack of 120 Tablets
Prescription Required *
Xeloda is a brand-name medication containing the active ingredient Capecitabine. It is manufactured by Roche Holding AG. Xeloda is available in the form of 500 mg tablets. This medication is commonly prescribed for the treatment of certain types of cancer, including breast cancer, colorectal cancer, and gastric cancer.
Composition:
Each Xeloda tablet contains 500 mg of Capecitabine as its active ingredient. The tablet also contains other inactive components such as microcrystalline cellulose, croscarmellose sodium, hypromellose, colloidal silicon dioxide, magnesium stearate, and talc.
Uses:
Xeloda is primarily used to treat cancer by inhibiting the growth and spread of cancer cells. It is particularly effective in treating breast cancer, colorectal cancer, and gastric cancer. Capecitabine is an oral chemotherapy drug that is converted into an active form in the body. This active form interferes with the replication of cancer cells, ultimately leading to their death and slowing down the progression of the disease.
Usage and Dosage:
Xeloda tablets should be taken orally with water, preferably within 30 minutes after a meal. The dosage and duration of treatment depend on several factors, including the type and stage of cancer, the patient’s overall health, and individual response to the medication. It is crucial to follow the specific instructions provided by the treating physician. Typically, the recommended starting dose of Xeloda is 1250 mg/m2 administered twice daily for two weeks, followed by a one-week rest period. This 3-week cycle is repeated for the recommended duration of treatment.
Storage Conditions:
Xeloda tablets should be stored at controlled room temperature (between 20°C and 25°C / 68°F and 77°F) in a tightly closed container, away from moisture and heat. It is important to keep this medication out of the reach of children and pets.
Mechanism of Action:
Capecitabine, the active ingredient in Xeloda, is a prodrug that is converted into an active form in the body. Once inside the body, Capecitabine undergoes enzymatic conversion, primarily in tumor cells, where it is transformed into fluorouracil (5-FU), which is an anti-cancer drug. 5-FU interferes with the replication of cancer cells by inhibiting the synthesis of DNA and RNA, ultimately leading to the death of cancer cells.
Contraindications:
Xeloda should not be used in individuals with a known hypersensitivity or allergy to Capecitabine or any other component of the medication. It is also contraindicated in patients with severe renal impairment. Xeloda is not recommended during pregnancy or while breastfeeding. It is important to discuss any pre-existing medical conditions, medications, or allergies with the treating physician before starting Xeloda.
Interactions:
Xeloda may interact with certain medications, potentially altering their effectiveness or increasing the risk of adverse effects. It is important to inform the treating physician about all current medications, including prescription drugs, over-the-counter medications, and herbal supplements. Medications that may interact with Xeloda include warfarin, phenytoin, leucovorin, and sorivudine.
Adverse Reactions:
Like all medications, Xeloda can cause adverse reactions in some individuals. The most common side effects include nausea, vomiting, diarrhea, abdominal pain, fatigue, and hand-foot syndrome (redness, swelling, and pain on the palms of hands and soles of feet). Other less common side effects may include loss of appetite, headache, dizziness, rashes, and hair loss. It is important to promptly report any side effects to the treating physician for appropriate management.
Conclusion:
Xeloda, containing Capecitabine 500 mg tablets, is a widely used medication for the treatment of various cancers, including breast cancer, colorectal cancer, and gastric cancer. Proper usage, dosage, and adherence to storage conditions are crucial to ensure its effectiveness. Before starting Xeloda, it is important to discuss any pre-existing medical conditions, allergies, or medications with the treating physician. Regular follow-up appointments and monitoring are essential to manage any potential adverse reactions or interactions.
Be the first to review “Buy Xeloda : Capecitabine 500 Mg Tablets Online” Cancel reply
Related products
Anti Cancer
Anti Cancer
Anti Cancer
Anti Cancer
Anti Cancer


 Buy Abstet : Abiraterone 250 Mg Tablets 120'S Online
Buy Abstet : Abiraterone 250 Mg Tablets 120'S Online 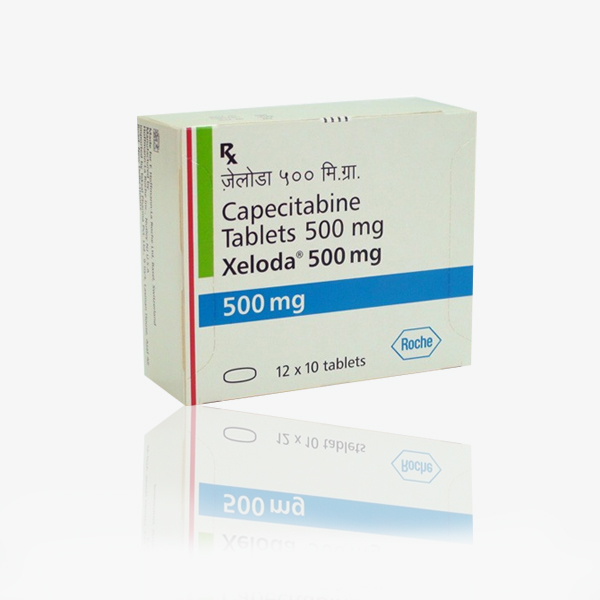







Reviews
There are no reviews yet.