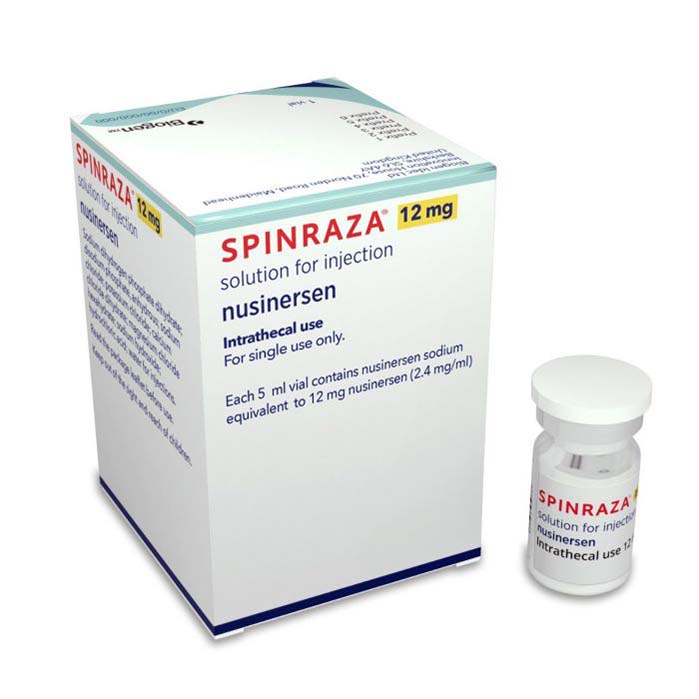
Buy Spinraza (Nusinersen) Online For Sale
DISEASE INDICATIONS: Spinal Muscular Atrophy
MANUFACTURER: Biogen Idec Ltd
USAGE: Intrathecal Injection
MEDICINE APPROVED BY:
European Medical Agency (EMA)
Food and Drug Administration (FDA)
Health Canada
Therapeutic Goods Administration (TGA)
Medsafe (NZ)
Spinraza is a gene therapy medication used to treat the rare genetic disorder Spinal Muscular Atrophy (SMA).
Spinraza (Nusinersen) is a breakthrough medication that has given new hope to patients suffering from a rare genetic disorder known as Spinal Muscular Atrophy (SMA). SMA is a progressive neuromuscular disease that affects the nerves and muscles responsible for movement and breathing. Spinraza is a revolutionary gene therapy drug that is used to treat this debilitating condition by replacing the missing or defective gene that causes SMA.
According to the National Institute of Neurological Disorders and Stroke, SMA is caused by a deficiency in a protein called SMN (Survival Motor Neuron). This protein is essential for the survival and function of the nerve cells that control muscle movement. When the SMN protein is absent or inadequate, the nerve cells die, and the muscles become weak and eventually atrophied.
Spinraza is designed to address this problem by delivering a functional of the SMN gene to the affected nerve cells. The drug works by targeting a specific RNA molecule called SMN2, which produces the SMN protein. Spinraza acts as a guide molecule and helps the cells produce more SMN protein from the SMN2 gene. By boosting the production of SMN protein, Spinraza helps to restore the function of the nerve cells and improve muscle strength.
The approval of Spinraza in 2016 marked a significant milestone in the treatment of SMA. Before Spinraza, there were no effective treatments approved by the FDA for SMA. Patients with SMA had no option but to undergo expensive medical procedures and therapies that could only slow down the progression of the disease. Spinraza has changed the lives of many SMA patients since its introduction.
The success of Spinraza has been proven in various clinical trials conducted on patients with SMA. In one such study, babies with a severe form of SMA who received Spinraza demonstrated significant improvements in motor function compared to those who did not receive treatment. The results of the study were so impressive that the FDA approved Spinraza as a first-line treatment option for patients with SMA.
Spinraza is administered through an injection into the cerebrospinal fluid surrounding the spinal cord. The drug is administered directly into the spinal cord to ensure that the gene therapy reaches the affected nerve cells. The treatment requires several injections over several months, depending on the severity of the patient’s condition and the progress of the therapy.
Although Spinraza has been a game-changer in the treatment of SMA, it still has some drawbacks. One of the most significant challenges with Spinraza is its cost. The drug comes with a hefty price tag of around $750,000 for the first year of treatment and around $375,000 for subsequent years. The high cost of Spinraza makes it inaccessible to many patients who cannot afford the medication.
Another challenge with Spinraza is that it requires multiple spinal injections over a long period, which can be painful and risky. The treatment may cause complications such as headaches, back pain, and spinal fluid leaks, which can be severe and require hospitalization.
Nonetheless, Spinraza has been a tremendous breakthrough in the treatment of SMA, and many patients are grateful for its availability. The drug has transformed the lives of many people who were once confined to a wheelchair or had limited mobility due to SMA. It has allowed them to regain their strength and independence, allowing them to live a more fulfilling life.
In conclusion, Spinraza (Nusinersen) has revolutionized the treatment of Spinal Muscular Atrophy (SMA) by providing a gene therapy that addresses the underlying cause of the disease. It has shown remarkable success in improving motor function and enhancing the quality of life of patients with SMA. Although Spinraza has its limitations, it represents a significant breakthrough in the treatment of genetic disorders and is a true example of how science can change lives.
| Package | 1 vial of 12 mg/5 mL |
|---|
Be the first to review “Buy Spinraza (Nusinersen) Online For Sale” Cancel reply
Related products
Neurology
Neurology
Neurology
Neurology
Neurology








Reviews
There are no reviews yet.