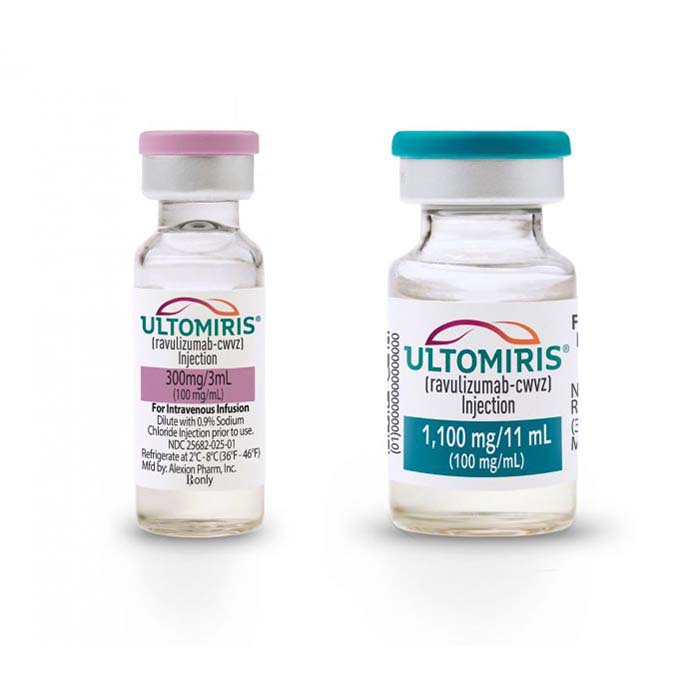
Buy Ultomiris (ravulizumab) Online For Sale
MANUFACTURER: Alexion Pharmaceuticals
USAGE: Intravenous
MEDICINE APPROVED BY:
European Medical Agency (EMA)
Food and Drug Administration (FDA)
Pharmaceuticals and Medical Devices Agency (PMDA)
Ultomiris (ravulizumab) is a medication used to treat paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS), both rare blood disorders that can cause life-threatening complications.
Ultomiris (ravulizumab) is a prescription medication used to treat adults and children with paroxysmal nocturnal hemoglobinuria (PNH) and adults and children with atypical hemolytic uremic syndrome (aHUS). These are rare and severe blood disorders that can cause life-threatening complications.
PNH is a condition that occurs when a person’s red blood cells break down at an accelerated rate, leading to a shortage of red blood cells and a lack of oxygen in the body’s tissues and organs. This can cause a variety of symptoms, including fatigue, shortness of breath, headaches, and abdominal pain. Left untreated, this condition can lead to severe anemia, blood clots, and kidney damage.
aHUS is another rare and serious condition that occurs when the body’s immune system mistakenly attacks and damages the body’s small blood vessels, leading to a shortage of red blood cells and platelets. This can cause a wide range of symptoms, including fatigue, weakness, and fluid retention. If left untreated, aHUS can lead to kidney failure, stroke, and even death.
Ultomiris works by blocking a protein called C5, which is part of the body’s immune system and helps propagate the inflammation and cell destruction seen in these diseases. By inhibiting C5, Ultomiris can help reduce the breakdown of red blood cells, prevent the formation of blood clots, and protect the body’s organs from damage.
Ultomiris is administered through intravenous infusion every eight weeks. Dosage and treatment frequency will depend on the patient’s condition and response to treatment. The medication should only be administered by a healthcare professional who is trained in the management of PNH and aHUS.
The effectiveness of Ultomiris has been demonstrated in clinical trials with PNH and aHUS patients, leading to regulatory approvals in many countries worldwide. In studies, Ultomiris showed a significant reduction in the frequency of transfusions required, from which the patients have to suffer, which requires humbling support for those struggling to fight this disease.
Common side effects of Ultomiris include headache, infections, diarrhea, nausea, and vomiting. Patients may also experience muscle pain, joint pain, and fever. Rare but severe side effects may include infusion-related reactions, allergic reactions, and meningococcal infections. Healthcare professionals must weigh the benefits and potential risks on a case-by-case basis.
In conclusion, Ultomiris (ravulizumab) is a breakthrough medication that offers hope to patients with paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). The medication works by blocking the C5 protein, preventing the breakdown of red blood cells, the formation of blood clots, and organ damage. Clinical trials demonstrate that Ultomiris is an effective therapy in the reduction of transfusions that are required. The medication is administered every eight weeks through intravenous infusion under the supervision of qualified healthcare professionals. However, as with other medications, there are some side effects, including possible serious complications such as allergic reactions and meningococcal infections. Despite these risks, Ultomiris offers patients an essential treatment option that can improve their quality of life and may even save lives.
| Package | One vial of 300 mg/30 ml (10 mg/ml), One vial of 1,100 mg/11 ml (100 mg/ml) |
|---|
3 reviews for Buy Ultomiris (ravulizumab) Online For Sale
Add a review Cancel reply
Related products
Haematology
Haematology
Haematology
Haematology
Haematology
Haematology
Haematology



Emily R. –
After switching to Ultomiris from a previous therapy, I noticed a big difference. The treatment controls my symptoms well, and I haven’t experienced the side effects I had before. The less frequent dosing is a huge plus, making it easier to keep up with treatment. I highly recommend it to anyone with PNH.
David M. –
Ultomiris fits perfectly into my busy lifestyle because I only need infusions every two months. Since starting treatment, I’ve had fewer symptoms and a much better quality of life. The medical staff administering the drug are always supportive, and I feel confident knowing this treatment is keeping my condition under control.
Sarah J. –
I was diagnosed with paroxysmal nocturnal hemoglobinuria (PNH) a couple of years ago, and Ultomiris has completely changed my life. The infusions are only every 8 weeks, which makes managing my condition so much easier compared to previous treatments. My symptoms have significantly decreased, and I have more energy than I’ve had in years.