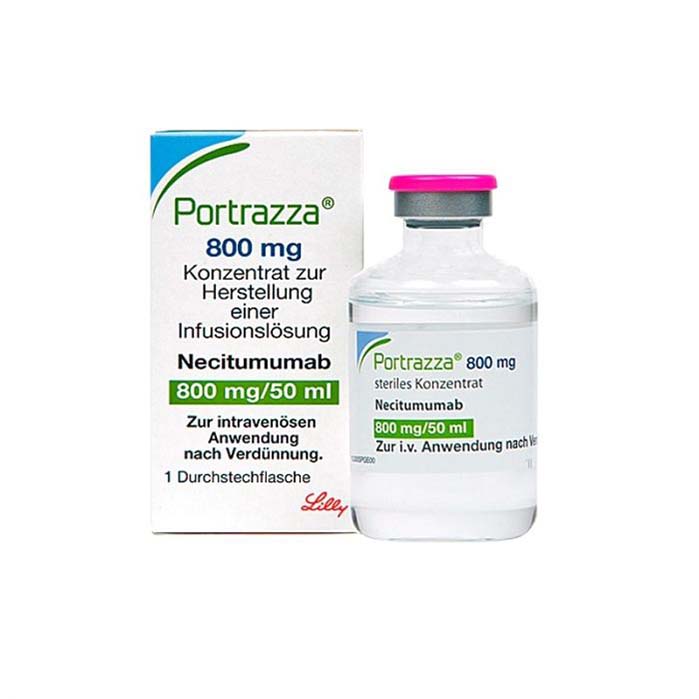
Buy Portrazza (necitumumab) Online For Sale
$7,033.00
DISEASE INDICATIONS: Lung Cancer
MANUFACTURER: Eli Lilly Nederland B.V.
USAGE: Intravenous
MEDICINE APPROVED BY:
European Medical Agency (EMA)
Food and Drug Administration (FDA)
Health Canada
Portrazza (necitumumab) is a medication approved for the treatment of advanced squamous non-small cell lung cancer. It is used in combination with the chemotherapies gemcitabine and cisplatin.
What is Portrazza (necitumumab) for?
Portrazza (necitumumab) is a prescription medication used for the treatment of advanced squamous non-small cell lung cancer (NSCLC) in combination with two other chemotherapy drugs, gemcitabine, and cisplatin, in patients who have not received prior chemotherapy for their advanced disease. It is a type of drug called a monoclonal antibody, which works by blocking a protein called epidermal growth factor receptor (EGFR) that is found on the surface of cancer cells, thereby slowing down or stopping their growth and spread.
How does Portrazza (necitumumab) work?
Portrazza (necitumumab) is a monoclonal antibody that binds to and inhibits the activity of the epidermal growth factor receptor (EGFR), a protein that is found on the surface of some cancer cells. By blocking the activity of EGFR, Portrazza slows down or stops the growth and spread of cancer cells. This slows down the progression of advanced squamous non-small cell lung cancer (NSCLC), in combination with gemcitabine and cisplatin, as the medication is able to target and inhibit the activity of the EGFR receptors that are present in these cancer cells. The inhibition of the EGFR receptors reduces the ability of the cancer cells to divide and spread, and can also lead to their destruction by the body’s immune system.
How is Portrazza (necitumumab) taken?
Portrazza (necitumumab) is administered as an intravenous (IV) infusion in a medical setting such as a hospital or clinic. It should only be given by a healthcare professional who is experienced in the administration of chemotherapy drugs. The recommended dose of Portrazza is 800 mg given over a 60-minute infusion, every two weeks until disease progression or unacceptable toxicity occurs. Portrazza is only given in combination with gemcitabine and cisplatin chemotherapy drugs. The dose and schedule may be adjusted by the healthcare provider based on the patient’s response and tolerability to the medication. Patients should follow the instructions of their healthcare provider and report any side effects or concerns that arise during treatment.
Are there any known side effects of Portrazza (necitumumab)?
Yes, there are some known side effects associated with Portrazza (necitumumab) use which you should be aware of. Some of the more common side effects seen in clinical trials of Portrazza include skin rash, hypomagnesemia, fatigue, vomiting, nausea, diarrhea, and breathing difficulty. Other less common side effects included infusion reactions, increased risk of blood clots, and decreased heart function.
Infrequent but serious side effects of Portrazza include severe allergic reactions such as anaphylaxis, skin symptoms such as Stevens-Johnson syndrome, toxic epidermal necrolysis, Other complications such as heart attack, stroke, low blood pressure, and lung fibrosis may occur. Discuss any concerns and possible side effects with your healthcare provider before starting treatment with Portrazza.
It is important to inform your healthcare provider immediately if you experience any of these symptoms or other unexpected effects while using Portrazza.
Clinical trials
The approval of Portrazza (necitumumab) was based on the SQUIRE study, which involved 1,093 patients with advanced squamous non-small cell lung cancer who received chemotherapies of gemcitabine and cisplatin with or without necitumumab. Those who received necitumumab lived an average of 1.6 months longer than those who received chemotherapy alone. The 1-year overall survival rate was also higher in patients receiving necitumumab. The addition of necitumumab resulted in a median progression-free survival of 5.7 months, compared to 5.5 months without its addition. However, necitumumab was found to be ineffective in treating patients with non-squamous NSCLC.
| Package | 1 vial of 800 mg/50mL (16mg/mL) |
|---|
Be the first to review “Buy Portrazza (necitumumab) Online For Sale” Cancel reply
Related products
Anti Cancer
Anti Cancer
Anti Cancer
Anti Cancer
Anti Cancer








Reviews
There are no reviews yet.