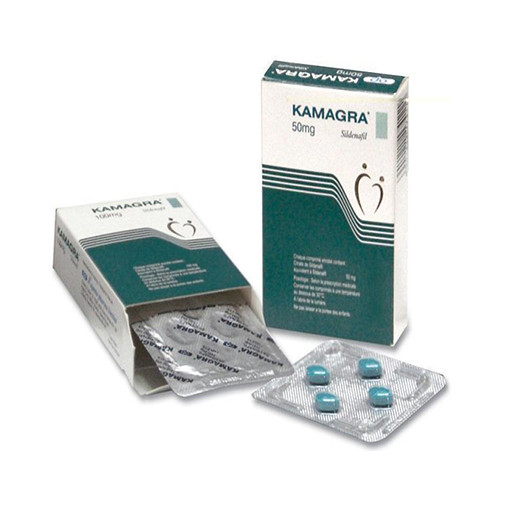
Buy KAMAGRA-50 Online
$5.90
CHARACTERISTICS
ACTIVE HALF-LIFE 4-5 HOURS
CLASSIFICATION PDE5 INHIBITOR
DOSAGE MEN 50 MG/DAY
ACNE NO
WATER RETENTION NO
HBR POSSIBLE
HEPATOTOXICITY NO
AROMATIZATION NO
ACTIVE SUBSTANCE SILDENAFIL
FORM 4 PILLS X 50 MG
MANUFACTURER AJANTA PHARMA
Kamagra-50 is a medication used to treat erectile dysfunction in men. It contains the active substance sildenafil, which belongs to a class of drugs called PDE5 inhibitors. The medication works by relaxing the blood vessels in the penis, allowing increased blood flow, which leads to an erection.
The active half-life of Kamagra-50 is approximately 4-5 hours. This means that the medication can remain effective in the body for up to 5 hours and can be taken up to once a day. The recommended dosage for men is 50 mg/day. The medication is available in the form of 4 pills, each containing 50 mg of sildenafil.
Kamagra-50 does not cause acne or water retention in the body. However, it is possible for some individuals to experience headaches, dizziness, and heartburn as side effects. HBR or hepatic blood flow reduction may also be a possible side effect in some individuals.
Kamagra-50 is not hepatotoxic, meaning it does not damage or cause harm to the liver. Furthermore, it does not aromatize, which means that it does not convert into estrogen in the body.
Kamagra-50 is manufactured by Ajanta Pharma, a reputable Indian pharmaceutical company. Overall, Kamagra-50 is a safe and effective medication for treating erectile dysfunction in men. However, it is important to consult with a healthcare provider before taking any medication to ensure safe and appropriate use.
Chemical name: 1-((3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo(4,3-d)pyrimidin-5-yl)-4-ethoxyphenyl)sulfonyl)-4-methylpiperazine citrate
Formula: C28H38N6O11S
Anabolic activity index: not a steroid
Androgenic activity index: not a steroid
Be the first to review “Buy KAMAGRA-50 Online” Cancel reply
Related products
Sexual Health
Sexual Health
Sexual Health
Sexual Health
Sexual Health
Sexual Health
Sexual Health
Sexual Health




Reviews
There are no reviews yet.