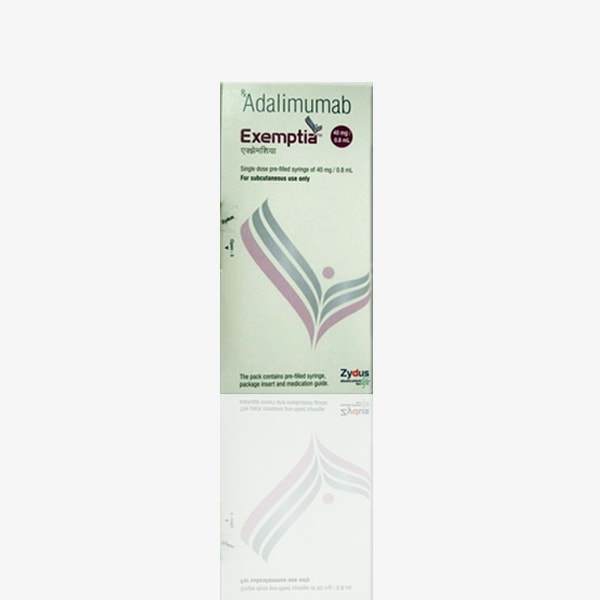
Buy Exemptia : Adalimumab 40 mg Injection Online
$194.85
Brand name: Exemptia
Active ingredient: Adalimumab
Strength: 40 mg/0.8 ml
Form: Injection
Packaging: Pack of Pre-Filled Syringe
Manufactured by: Zydus
Prescription Required
Exemptia (adalimumab) is a biologic medication used to treat various inflammatory conditions such as rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, and ulcerative colitis. Adalimumab is a type of monoclonal antibody that works by targeting and blocking tumor necrosis factor alpha (TNFα), which is a cell signaling protein that causes inflammation in the body.
Composition:
Each Exemptia 40 mg injection contains adalimumab (40 mg) as the active ingredient, as well as mannitol, sucrose, sodium phosphate, polysorbate 80, sodium chloride, and water for injection as excipients.
Uses:
Exemptia is used to treat various inflammatory conditions, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, and ulcerative colitis. It can also be used to treat other autoimmune conditions such as hidradenitis suppurativa and non-infectious uveitis.
Usage and Dosage:
Exemptia is administered by subcutaneous injection and is available in pre-filled syringes or auto-injectors. The dosage and frequency of administration depend on the condition being treated. For adults with rheumatoid arthritis or psoriatic arthritis, the recommended dose is 40 mg injected subcutaneously every other week. For ankylosing spondylitis or Crohn’s disease, the recommended dose is 40 mg injected subcutaneously every other week. For ulcerative colitis, the initial dose is 160 mg (four 40 mg injections) followed by 80 mg (two 40 mg injections) two weeks later and then 40 mg every other week.
Storage Conditions:
Exemptia should be stored in the refrigerator at 2°C-8°C (36°F-46°F). Do not freeze the medication. Once removed from the refrigerator, the injection should be allowed to reach room temperature before use. If it is not used within two hours, it should be discarded.
Mechanism of action:
Adalimumab is a monoclonal antibody that targets and binds to tumor necrosis factor alpha (TNFα), a cytokine that is involved in the inflammatory response in the body. By binding to TNFα, adalimumab blocks its activity, which leads to a reduction in inflammation and associated symptoms such as pain and swelling.
Contraindications:
Exemptia is contraindicated for use in individuals who are hypersensitive to adalimumab or any of its components. It should not be used in patients with active infections, including tuberculosis and sepsis. Exemptia should not be used in patients with congestive heart failure. It is not recommended for use in patients with multiple sclerosis or other demyelinating disorders.
Interactions:
Exemptia may interact with other immunosuppressive medications, increasing the risk of infection. It may also interfere with the effectiveness of vaccines. Patients should avoid live vaccines while receiving treatment with Exemptia.
Side Effects:
The most common side effects of Exemptia include injection site reactions (pain, redness, swelling), headache, upper respiratory tract infection, and nausea. Other less common side effects may include allergic reactions, infections, blood disorders, liver problems, and nerve disorders. Rare cases of lymphoma and other malignancies have been reported in patients receiving TNF-blocking agents, including Exemptia. Patients should be monitored for signs of infection, including fever, chills, and coughing. If any severe side effects are experienced, patients should seek immediate medical attention.
Be the first to review “Buy Exemptia : Adalimumab 40 mg Injection Online” Cancel reply
Related products
Osteoporosis Arthritis
Osteoporosis Arthritis
Buy Prolia (denosumab) Injection 1 pre-filled syringe 60mg/ml Online For Sale
Osteoporosis Arthritis
Osteoporosis Arthritis
Osteoporosis Arthritis
Osteoporosis Arthritis
Osteoporosis Arthritis
Osteoporosis Arthritis
Buy Scapho : Secukinumab 150 mg/ml Injection Online For Sale





Reviews
There are no reviews yet.