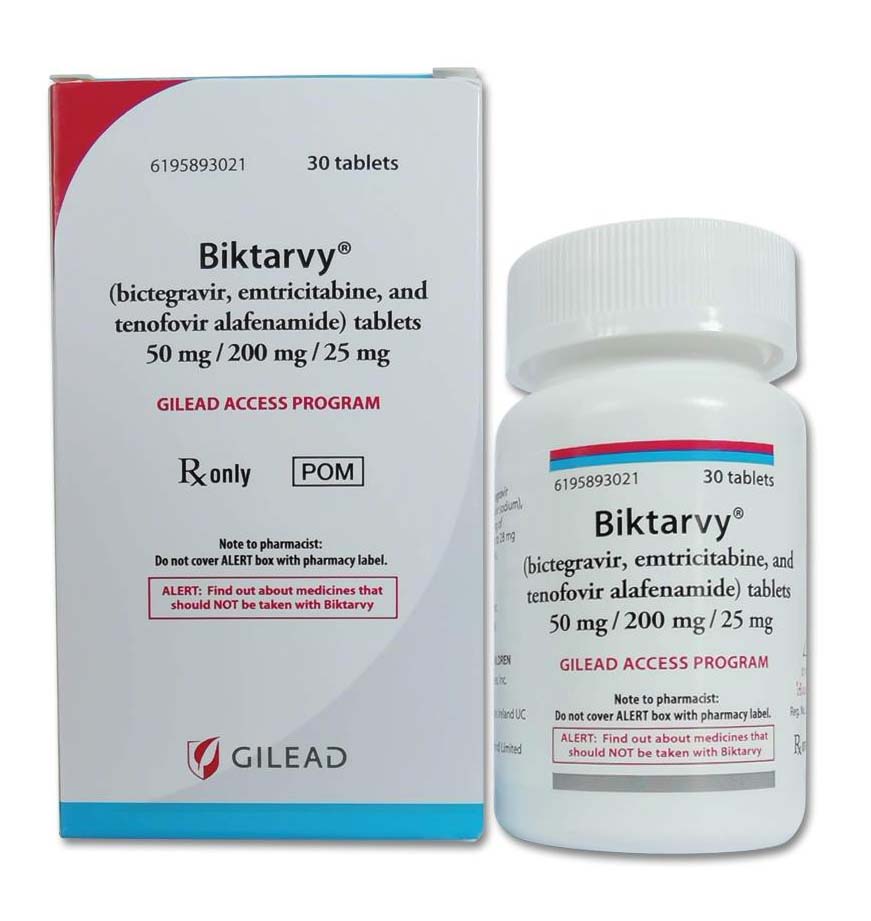
Buy Biktarvy (bictegravir, emtricitabine, tenofovir alafenamide) Gilead Sciences Online
$1,060.00
DISEASE INDICATIONS: HIV/AIDS
MANUFACTURER: Gilead Sciences International Limited
USAGE: Oral
MEDICINE APPROVED BY:
European Medical Agency (EMA)
Food and Drug Administration (FDA)
Health Canada
Therapeutic Goods Administration (TGA)
Biktarvy is a medication approved by the FDA for the treatment of HIV-1. It contains three drugs in a single tablet – tenofovir alafenamide, emtricitabine, and bictegravir – which work together to reduce the viral load of HIV-1 and improve the immune system of those living with the virus.
Biktarvy is a pharmaceutical drug that has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of HIV-1. The medication is produced in the form of a tablet, and it includes three drugs that are combined into a single pill. These three drugs are tenofovir alafenamide (TAF), emtricitabine (FTC), and bictegravir (BIC).
Tenofovir alafenamide is an antiviral drug that belongs to the nucleos(t)ide reverse transcriptase inhibitor (NRTI) class. Tenofovir targets reverse transcriptase, which is an enzyme that is necessary for the replication of the HIV virus. By inhibiting the activity of reverse transcriptase, tenofovir can prevent the virus from multiplying and spreading in the body.
Emtricitabine is a cytosine analogue that also works by inhibiting the activity of reverse transcriptase. By blocking the activity of this enzyme, emtricitabine can prevent the virus from replicating and ultimately reduce the viral load in the body.
Bictegravir is an integrase strand transfer inhibitor (INSTI) that blocks the activity of integrase, which is another enzyme that is necessary for the replication of the HIV virus. By inhibiting integrase, bictegravir can prevent the virus from integrating its genetic material into the DNA of the host cell. This helps to further limit the replication of the virus and reduce the amount of virus in the bloodstream.
Together, these three drugs work synergistically to reduce the viral load of HIV-1 and improve the immune system of those living with the virus.
Biktarvy was first approved by the FDA in 2018, and it quickly gained traction in the medical community due to its effectiveness and safety. It has been shown to be highly effective at reducing viral loads and raising CD4 cell counts in clinical trials. In addition, it has a low incidence of side effects, which makes it an attractive option for those living with HIV.
Biktarvy has several advantages over other HIV medications. Firstly, it is a once-daily tablet that can be taken with or without food, which makes it very convenient for those living with HIV. Secondly, it has a high barrier to resistance, which means that it is less likely to develop drug-resistant strains of HIV over time. This is especially important in the long-term management of HIV, as drug-resistant strains can limit treatment options and make the virus more difficult to manage.
Finally, Biktarvy is also effective for those with underlying health conditions, such as renal impairment or hepatitis B. This is because it contains tenofovir alafenamide, which is associated with a lower risk of kidney and bone toxicity compared to other HIV medications.
However, like any medication, Biktarvy does have some potential side effects. The most common side effects reported in clinical trials were diarrhea, nausea, and headache. In addition, it is important to note that Biktarvy is not a cure for HIV, and it is still necessary for those living with the virus to take the medication on a daily basis to maintain its efficacy and prevent the replication of the virus.
In conclusion, Biktarvy is a groundbreaking medication that has revolutionized the treatment of HIV. Its combination of three antiretroviral drugs delivered in a single tablet has simplified HIV treatment and improved patient outcomes. Its high level of efficacy, safety, and convenience has made it a popular choice among medical professionals and those living with HIV alike. However, it is important to continue researching and developing new treatments for HIV in order to ultimately find a cure for this devastating virus.
| Package | 50 mg bictegravir, 200 mg emtricitabine, 25 mg tenofovir alafenamide x 30 film-coated tablets |
|---|








Reviews
There are no reviews yet.