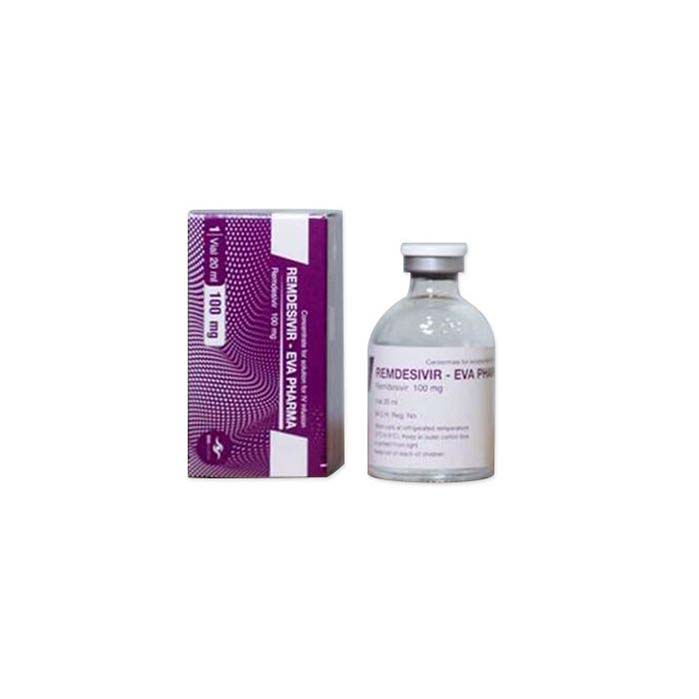
Buy Veklury (remdesivir) Online For Sale
DISEASE INDICATIONS: COVID-19
MANUFACTURER: Gilead Sciences International Limited
USAGE: Intravenous
MEDICINE APPROVED BY:
European Medical Agency (EMA)
Food and Drug Administration (FDA)
Health Canada
Pharmaceuticals and Medical Devices Agency (PMDA)
Therapeutic Goods Administration (TGA)
Veklury (remdesivir) is an antiviral medication used to treat COVID-19 in hospitalized adolescents and adults with pneumonia and oxygen requirements.
Veklury (remdesivir) is an antiviral medication that has received approval for the treatment of COVID-19. Its composition includes the active drug remdesivir, which is a nucleotide analogue prodrug that inhibits viral RNA replication. It is available as a sterile lyophilized powder in vials for injection.
Uses:
Veklury is used to treat patients with severe COVID-19 who require hospitalization and supplemental oxygen. It has shown efficacy in shortening the duration of hospitalization and reducing the need for mechanical ventilation in some patients.
Usage and Dosage:
Veklury is administered intravenously over a period of 30-120 minutes, depending on the dose. The recommended dosage for adults and pediatric patients weighing at least 40 kg is a loading dose of 200 mg on day 1, followed by a maintenance dose of 100 mg daily for 4-9 days. The course of treatment may be extended based on the patient’s clinical condition.
Storage Conditions:
Veklury should be stored in its original packaging at 20-25°C (68-77°F), with excursions permitted between 15-30°C (59-86°F). The vials should be protected from light, and any unused portions should be discarded after reconstitution.
Mechanism of Action:
Veklury works by inhibiting the RNA-dependent RNA polymerase enzyme that is critical for viral replication. It is converted into an active form that competes with adenosine triphosphate (ATP) for incorporation into nascent viral RNA chains, leading to premature termination of RNA synthesis.
Contraindications:
Veklury is contraindicated in patients with a known hypersensitivity to remdesivir or any of its components. It should not be used concurrently with other antiviral medications that have overlapping mechanisms of action.
Interactions:
There are no known drug interactions with Veklury, but caution should be exercised when administering concomitant medications that may affect renal function or electrolyte levels.
Side Effects:
Common side effects of Veklury include nausea, vomiting, diarrhea, elevated liver enzymes, and hypotension. It may also cause infusion-related reactions such as fever, chills, and pruritus. In rare cases, it may lead to acute kidney injury or liver injury. Healthcare providers should monitor patients closely for any signs of adverse reactions.
In conclusion, Veklury (remdesivir) is an FDA-approved antiviral medication used to treat severe COVID-19 in hospitalized patients requiring supplemental oxygen. It works by inhibiting viral RNA replication and has shown efficacy in shortening the duration of hospitalization and reducing the need for mechanical ventilation. However, caution should be exercised when administering concomitant medications and monitoring for any signs of adverse reactions.
| Package | 100 mg, lyophilized powder |
|---|
Be the first to review “Buy Veklury (remdesivir) Online For Sale” Cancel reply
Related products
Infectiology
Infectiology
Infectiology
Dermatology
Infectiology






Reviews
There are no reviews yet.