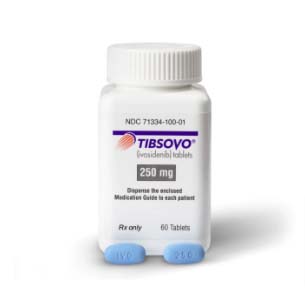
Buy Tibsovo (ivosidenib) Online
$23,691.00
Disease Indications: Leukemia, Bile Duct Cancer
Manufacturer: Agios Pharmaceuticals, Inc.
Usage: Oral
Medicine approved by:
Food and Drug Administration (FDA)
Tibsovo (ivosidenib) is designed to treat two conditions: relapsed or refractory acute myeloid leukemia (AML) with an IDH1 mutation, and previously treated, locally advanced or metastatic cholangiocarcinoma (bile duct cancer) with the same mutation.
Overview: Tibsovo (ivosidenib) is a medication developed by Agios Pharmaceuticals, Inc. It is specifically indicated for the treatment of two distinct conditions: leukemia and bile duct cancer. Tibsovo belongs to a class of drugs known as IDH1 inhibitors, targeting mutations in the isocitrate dehydrogenase 1 (IDH1) enzyme.
Disease Indications:
- Leukemia: Tibsovo is approved for the treatment of relapsed or refractory acute myeloid leukemia (AML) with an IDH1 mutation. This genetic alteration is found in a subset of AML cases and contributes to abnormal cell proliferation.
- Bile Duct Cancer (Cholangiocarcinoma): Tibsovo is also indicated for adults with previously treated, locally advanced or metastatic cholangiocarcinoma (bile duct cancer) with an IDH1 mutation. This mutation plays a role in the development and progression of bile duct cancer.
Manufacturer:
- Agios Pharmaceuticals, Inc.: Tibsovo is manufactured by Agios Pharmaceuticals, a biopharmaceutical company specializing in therapies targeting cancer metabolism and rare genetic disorders.
Usage:
- Oral Administration: Tibsovo is administered orally, typically in the form of tablets or capsules. The dosage and duration of treatment are determined based on the patient’s medical condition, response to therapy, and other relevant factors as assessed by healthcare providers.
Mechanism of Action: Tibsovo works by selectively inhibiting the mutant IDH1 enzyme, which is involved in abnormal cellular metabolism and proliferation in leukemia and bile duct cancer. By targeting this specific mutation, Tibsovo helps to disrupt cancer cell growth and survival mechanisms.
Clinical Considerations:
- Monitoring: Regular monitoring of liver function tests and blood counts may be necessary during treatment with Tibsovo to manage potential side effects.
- Side Effects: Common side effects may include nausea, fatigue, diarrhea, increased bilirubin levels, and differentiation syndrome (a potentially serious condition requiring medical attention).
Precautions:
- Pregnancy and Breastfeeding: Tibsovo may cause fetal harm and is not recommended during pregnancy or breastfeeding. Patients should discuss family planning with their healthcare provider before starting treatment.
- Drug Interactions: Tibsovo may interact with other medications, including those metabolized by CYP3A4 enzymes. It is important to inform healthcare providers about all current medications, supplements, and herbal products.
In conclusion, Tibsovo (ivosidenib) represents a targeted therapy option for patients with specific forms of leukemia and bile duct cancer characterized by an IDH1 mutation. Its oral administration and mechanism of action make it a valuable treatment choice, offering a tailored approach to managing these challenging diseases under medical supervision.
| Package | 60 tablets of 250 mg |
|---|
Be the first to review “Buy Tibsovo (ivosidenib) Online” Cancel reply
Related products
New medicines
New medicines
New medicines
New medicines
New medicines






Reviews
There are no reviews yet.