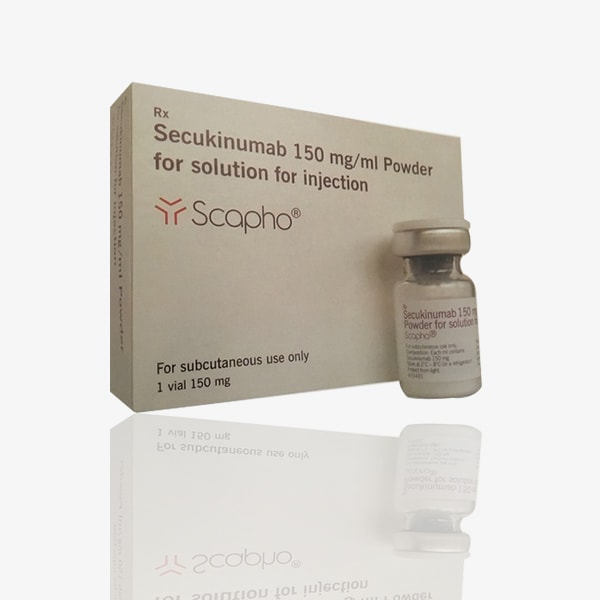
Buy Scapho : Secukinumab 150 mg/ml Injection Online For Sale
$235.00
Brand Name : Scapho
Composition : Secukinumab
Manufactured by : Novartis India Ltd.
Imported by : Sandoz Private Limted India
Strength : 150 mg/ml
Form : Injection
Packing : Pack of 1 Vial
Prescription Required *
Scapho: Secukinumab 150 mg/ml Injection is a prescription medication used to treat moderate to severe plaque psoriasis in adults. This medication is also used for psoriatic arthritis and ankylosing spondylitis. Scapho is a biologic drug that specifically inhibits interleukin-17A, a cytokine that plays a key role in the pathogenesis of autoimmune disorders.
Composition:
Scapho contains secukinumab as the active ingredient, which is a human monoclonal antibody generated in Chinese hamster ovary cells. The injection also contains L-histidine, L-histidine monohydrochloride monohydrate, polysorbate 80, water for injection, and succinic acid as inactive components.
Uses:
Scapho is used to reduce the symptoms of moderate to severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis in adults. It is specifically designed to target and prevent interleukin-17A from causing inflammation in the body.
Dosage:
Scapho is available as a 150 mg/mL strength solution for injection. The dosage prescribed is 150 mg, which is administered subcutaneously once every four weeks. The dosage may be adjusted based on the patient’s response to treatment. Patients are advised to read the medication guide that comes with the package carefully before initiating the treatment.
Instructions:
Scapho is a prescription drug that must be administered by a healthcare professional. It is given by subcutaneous injection, usually in the thigh or abdomen. The injection site should be alternated to avoid skin irritation. Patients should avoid touching or rubbing the injection site after administration. It is important to follow the recommended dosage, administration frequency, and storage instructions.
Storage Conditions:
Scapho should be stored in the refrigerator at 2°C to 8°C (36°F to 46°F) and kept protected from light. The medication should not be frozen, shaken or used after the expiration date.
Advantages:
Scapho is an effective treatment option for moderate to severe psoriasis, psoriatic arthritis, and ankylosing spondylitis. Compared to previous psoriasis treatments, including biologics, it has demonstrated a lower incidence of adverse events, therefore, it has been regarded as a safer drug.
Mechanism of Action:
Interleukin-17A is a cytokine that plays a key role in inflammation and the development of autoimmune disorders. Scapho works by specifically inhibiting this protein, preventing it from binding to its receptors in the body and reducing inflammation. By reducing inflammation and preventing further damage to organs in the body, patients can experience a significant reduction in symptoms.
Contraindications:
Scapho is contraindicated in patients with a history of serious allergic reactions to secukinumab or any of its components, active tuberculosis or other severe infections, or have immune system disorders. Patients must be screened for tuberculosis before initiating the treatment.
Side Effects:
The common side effects of Scapho include infections of the upper respiratory tract, gastrointestinal disorders, headache, and injection site reactions such as redness, swelling, or itching. Serious side effects such as severe infections, gastrointestinal disorders, and depression may also occur. Patients should seek medical attention immediately if they experience any side effects.
Conclusion:
Scapho: Secukinumab 150 mg/ml Injection is an effective treatment option for moderate to severe plaque psoriasis in adults. Studies have shown that Scapho is well tolerated and has a safer side effect profile compared to previous biologic drugs. It works by inhibiting the action of interleukin-17A, reducing inflammation, and improving symptoms. Patients are advised to follow the recommended dosage, administration frequency, and storage instructions.
Be the first to review “Buy Scapho : Secukinumab 150 mg/ml Injection Online For Sale” Cancel reply
Related products
Osteoporosis Arthritis
Osteoporosis Arthritis
Osteoporosis Arthritis
Osteoporosis Arthritis
Osteoporosis Arthritis
Osteoporosis Arthritis
Osteoporosis Arthritis
Osteoporosis Arthritis







Reviews
There are no reviews yet.