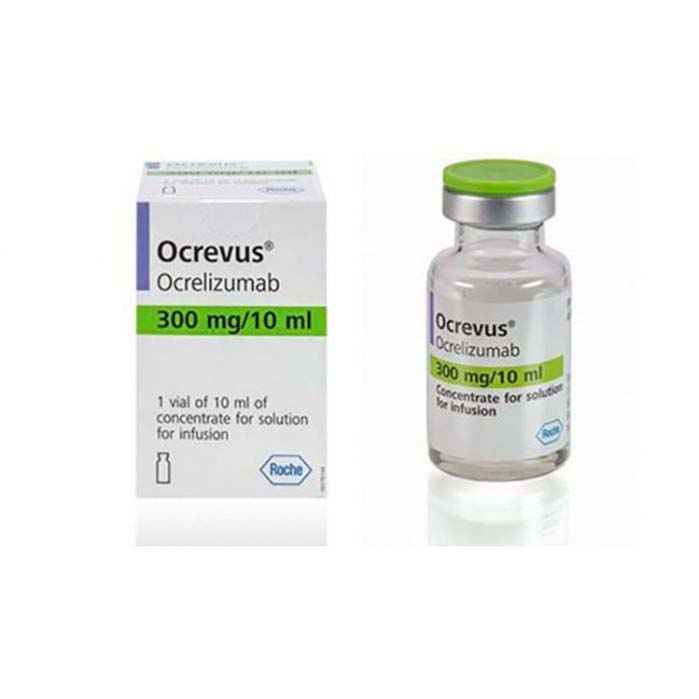
Buy Ocrevus (ocrelizumab) Online For Sale
$5,500.00
Disease Indications: Multiple Sclerosis
Manufacturer: Roche Registration Limited
Usage: Intravenous
Medicine approved by:
European Medical Agency (EMA)
Food and Drug Administration (FDA)
Health Canada
Therapeutic Goods Administration (TGA)
Medsafe (NZ)
Ocrevus (ocrelizumab) is a medication used to treat multiple sclerosis. It targets CD20 protein found on the surface of certain immune cells, reducing attacks, and progression of the disease. It is administered as an intravenous infusion every 6 months and has been shown to significantly reduce relapse rates in people with relapsing-remitting MS, slow disability progression in those with relapsing and progressive MS. It is being investigated for potential use in other autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus.
Description
Ocrevus (ocrelizumab) is a medication that is used to treat multiple sclerosis (MS). It was developed by Genentech, a subsidiary of Roche, and was approved by the US Food and Drug Administration (FDA) in March 2017.
MS is a chronic disease that affects the central nervous system (CNS), which includes the brain, spinal cord, and optic nerves. It occurs when the immune system mistakenly attacks myelin, a substance that covers and protects nerve fibers. This can lead to a range of symptoms, including fatigue, difficulty walking, numbness and tingling, vision problems, and cognitive impairment.
Ocrevus is a monoclonal antibody that targets a protein called CD20, which is found on the surface of certain immune cells. By binding to CD20, Ocrevus depletes these immune cells, including B cells, which are thought to play a key role in MS. This can reduce the number of attacks or relapses experienced by people with MS, as well as slow the progression of disability over time.
Ocrevus is administered as an intravenous infusion (IV) every 6 months. The first infusion is given in two parts, with the second part following 2 weeks after the first. Subsequent infusions are given as a single dose. The infusion takes several hours to complete and is typically administered in a hospital or clinic setting.
Clinical trials have shown that Ocrevus can significantly reduce the relapse rate in people with relapsing-remitting MS, the most common form of the disease. In addition, Ocrevus has been shown to slow the progression of disability in people with both relapsing and primary progressive MS, a more severe and rare form of the disease.
Like all medications, Ocrevus can cause side effects. The most common side effects include infusion-related reactions, which can include fever, chills, headache, nausea, and itching. In addition, Ocrevus can increase the risk of infections, including serious infections such as pneumonia and meningitis. People taking Ocrevus are advised to inform their healthcare provider immediately if they experience any symptoms of infection, such as fever, cough, or difficulty breathing.
Ocrevus is contraindicated in people with active hepatitis B virus (HBV) infection, as well as those who have had a severe allergic reaction to Ocrevus or any of its components. Before starting treatment with Ocrevus, healthcare providers should test patients for HBV and monitor them for signs of HBV reactivation during treatment and for several months thereafter.
In addition to its use in MS, Ocrevus is being investigated for its potential to treat other autoimmune diseases, including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Early studies have shown promising results, but further research is needed before Ocrevus can be approved for these indications.
Ocrevus represents an important advance in the treatment of MS, providing a new option for people with both relapsing and primary progressive forms of the disease. While it is not a cure, it has been shown to significantly reduce relapses and slow the progression of disability over time. As with any medical treatment, people considering Ocrevus should discuss the risks and benefits with their healthcare provider to determine if it is right for them.
Additional information
| Package | 1 vial of 300 mg/mL |
|---|

Reviews
There are no reviews yet.