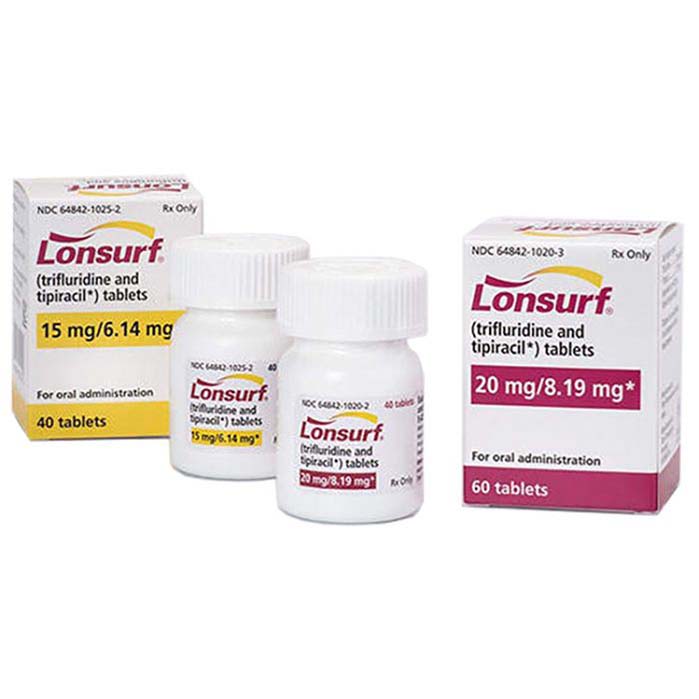
Buy Lonsurf (trifluridine/tipiracil) Online For Sale
From $593.00
DISEASE INDICATIONS: Colorectal Cancer
MANUFACTURER: Les Laboratoires Servier
USAGE: Oral
MEDICINE APPROVED BY:
European Medical Agency (EMA)
Food and Drug Administration (FDA)
Health Canada
Therapeutic Goods Administration (TGA)
Lonsurf (trifluridine/tipiracil) is a chemotherapy medication that has been approved by the Food and Drug Administration (FDA) in the USA, European Medical Agency (EMA) in the European Union, and Therapeutic Goods Administration (TGA) in Australia for the treatment of metastatic colorectal cancer in previously treated patients.
What is Lonsurf (trifluridine/tipiracil) for?
Lonsurf (trifluridine/tipiracil) is used to treat adult patients with advanced or metastatic colorectal cancer that has spread to other parts of the body and is no longer responding to standard therapies. It is a combination of two drugs: trifluridine, a nucleoside metabolic inhibitor, and tipiracil, a thymidine phosphorylase inhibitor. Trifluridine works by inhibiting the growth and spread of cancer cells, while tipiracil enhances the effectiveness of trifluridine by ensuring that it remains in the body for a longer period of time. Lonsurf is used as a last resort when other conventional treatments have failed.
How does Lonsurf (trifluridine/tipiracil) work?
Lonsurf (trifluridine/tipiracil) is a combination medication that works by targeting and inhibiting the growth of cancerous cells. Trifluridine is a nucleoside analog that works by incorporating itself into the DNA of dividing cancer cells, leading to the destruction of the cancerous cells. Tipiracil is known as a thymidine phosphorylase inhibitor, which makes it easier for trifluridine to enter the cancer cells. Tipiracil also blocks the degradation of trifluridine in the body by inhibiting the production of thymidine, which breaks down trifluridine into inactive metabolites. In combination, these two drugs help to slow the growth and spread of cancer cells, ultimately leading to their death.
How do I take Lonsurf (trifluridine/tipiracil)?
The recommended standard dosage for Lonsurf (trifluridine/tipiracil) is to take 35 mg/m2/dose orally twice daily on Days 1 through 5 and Days 8 through 12 of each 28-day cycle. It is important to take the medication within one hour after completing morning and evening meals. For more detailed information on dosage and administration, please refer to the official prescribing information listed in our references section [3,4,6]. Healthcare providers are advised to conduct complete blood counts prior to starting each treatment cycle of Lonsurf and to monitor patients throughout treatment, as myelosuppression may occur due to trifluridine/tipiracil’s severe decrease in blood cell and platelet production [1,4]. Note that personal dosing information should be obtained from one’s treating doctor.
Are there any side effects of Lonsurf (trifluridine/tipiracil)?
Yes, Lonsurf (trifluridine/tipiracil) can have several side effects. Some common side effects of Lonsurf include vomiting, nausea, fatigue, decreased appetite, constipation, abdominal pain, fever, weakness, anemia, and thrombocytopenia. These side effects are usually mild to moderate in intensity, but they can be severe in some cases. Other less common side effects of Lonsurf include headache, dizziness, shortness of breath, chest pain, coughing, and skin rash.
Lonsurf can also weaken the body’s immune system and increase the risk of infections. It can also cause a decrease in white blood cell, red blood cell, and platelet counts, which may require medical attention.
People with a history of allergic reactions to trifluridine or tipiracil should not take Lonsurf. In rare cases, Lonsurf has been associated with serious side effects such as liver problems, heart attack, and kidney problems.
It is important to report any side effects or concerns to your healthcare provider immediately. Your healthcare provider can advise you on how to manage and treat the side effects of Lonsurf.
Clinical trials
The Food and Drug Administration (FDA) in the USA, European Medical Agency (EMA) in the European Union, and Therapeutic Goods Administration (TGA) in Australia approved Lonsurf (trifluridine/tipiracil) for the treatment of metastatic colorectal cancer in previously treated patients based on a randomized, double-blind phase III study (RECOURSE). This study consisted of 800 patients with previously treated metastatic colorectal cancer who were administered either Lonsurf plus best supportive care or placebo plus best supportive care. Patients were treated until they experienced disease progression or unacceptable toxicity [3,4,6,7].
The study measured major efficacy outcomes such as overall survival (OS) and progression-free survival (PFS). The median OS was 7.1 months [95% CI: 6.5, 7.8], and the rate of deaths was 68.2% with Lonsurf (trifluridine/tipiracil). In contrast, the median OS was 5.3 months [95% CI: 4.6, 6.0], and the rate of deaths was 78.9% with placebo. The median PFS was 2.0 months [95% CI: 1.9, 2.1], and the rate of progressions or deaths was 88.4% with Lonsurf (trifluridine/tipiracil). Regarding placebo, the median PFS was 1.7 months [95% CI: 1.7, 1.8], and the rate of progressions or deaths was 94.4% [3,4,6,7].
| Package | 20 tablets of 15 mg/6.14 mg, 60 tablets of 15 mg/6.14 mg, 20 tablets of 20 mg/8.19 mg, 60 tablets of 20 mg/8.19 mg |
|---|
Be the first to review “Buy Lonsurf (trifluridine/tipiracil) Online For Sale” Cancel reply
Related products
Anti Cancer
Anti Cancer
Anti Cancer
Anti Cancer






Reviews
There are no reviews yet.