Buy Cuprior (trientine) Online
$8,538.00
Disease Indications: Wilson’s disease
Manufacturer: GMP-Orphan (GMPO)
Usage: Intravenous
Medicine approved by:
European Medical Agency (EMA)
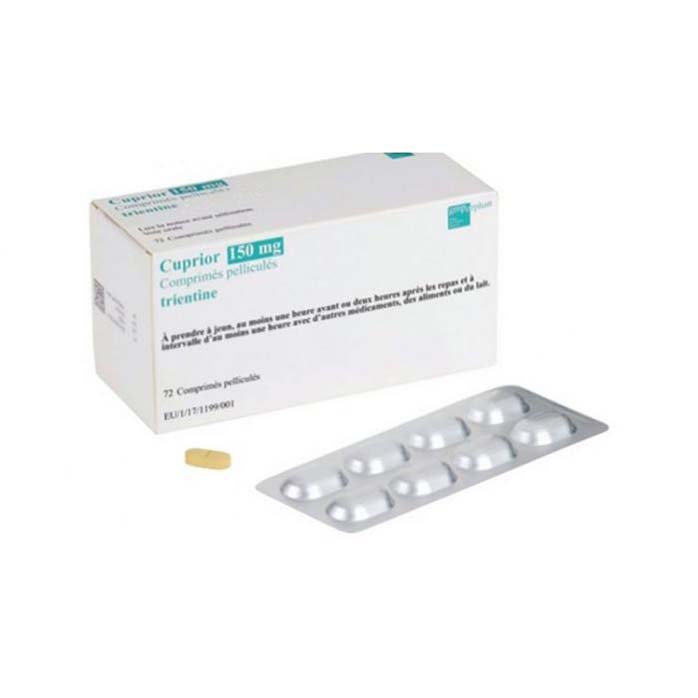
Cuprior, manufactured by GMP-Orphan (GMPO), is a vital intravenous medication used to treat Wilson’s disease, a genetic disorder causing excessive copper accumulation in the body.
Introduction: Cuprior, manufactured by GMP-Orphan (GMPO), is a vital medication used in the management of Wilson’s disease, a rare genetic disorder that results in excessive accumulation of copper in the body. Cuprior contains the active substance Trientine, which acts as a chelating agent, helping to remove excess copper and prevent further copper buildup in vital organs. This medication is administered intravenously, providing an effective treatment option for patients with Wilson’s disease.
Disease Indications: Cuprior is specifically indicated for the treatment of Wilson’s disease. Wilson’s disease is an inherited disorder characterized by the body’s inability to properly eliminate excess copper. This leads to the accumulation of copper in the liver, brain, and other vital organs, causing a range of symptoms including liver disease, neurological deficits, and psychiatric issues. Effective copper chelation therapy is essential to manage this condition and prevent serious complications.
Active Substance: The active substance in Cuprior is Trientine. Trientine is a chelating agent that binds to copper, facilitating its excretion from the body and thereby reducing copper levels in the tissues. This mechanism helps prevent the toxic effects of copper accumulation in patients with Wilson’s disease.
Formulation and Packaging: Cuprior is formulated for intravenous administration, ensuring efficient delivery of Trientine directly into the bloodstream. This method of administration is particularly beneficial for patients who require rapid reduction of copper levels or who may have difficulty with oral medications. The product is available in a form suitable for hospital or clinical use, ensuring proper handling and administration by healthcare professionals.
Usage and Administration: Cuprior should be administered intravenously by a healthcare professional. The dosage and frequency of administration depend on the individual patient’s condition, severity of the disease, and response to treatment. Typically, the medication is infused slowly to ensure optimal absorption and minimize potential side effects. Healthcare providers will determine the appropriate treatment regimen and monitor the patient’s progress closely.
Mechanism of Action: Trientine, the active ingredient in Cuprior, works by chelating copper. It binds to free copper ions in the bloodstream, forming a complex that is then excreted in the urine. This process helps to reduce the copper burden in the body, thereby alleviating the symptoms of Wilson’s disease and preventing further damage to organs.
Safety Considerations: Cuprior is generally well-tolerated when administered as prescribed. However, like all medications, it may cause side effects in some patients. Common side effects include gastrointestinal disturbances such as nausea and vomiting, as well as potential allergic reactions. More severe side effects, such as hematological abnormalities or renal impairment, require immediate medical attention. Regular monitoring of copper levels, liver function, and renal function is essential during treatment to ensure safety and efficacy.
Contraindications: Cuprior is contraindicated in patients with a known hypersensitivity to Trientine or any other component of the medication. It should not be used in patients with severe renal impairment unless deemed necessary by a healthcare provider and under strict medical supervision. Additionally, caution is advised in patients with pre-existing hematological disorders or those who are pregnant or breastfeeding, as Trientine can affect copper and iron metabolism.
Manufacturer: Cuprior is manufactured by GMP-Orphan (GMPO), a pharmaceutical company dedicated to developing and providing treatments for rare diseases. GMPO ensures the highest standards of quality and safety in the production of Cuprior, making it a reliable choice for the management of Wilson’s disease.
Conclusion: Cuprior (Trientine) by GMP-Orphan is a critical medication for patients with Wilson’s disease, offering effective copper chelation therapy to manage this challenging condition. Its intravenous administration ensures rapid and efficient copper removal, helping to alleviate symptoms and prevent complications. Adherence to prescribed dosages, regular monitoring by healthcare professionals, and ongoing medical supervision are essential to maximize the benefits of Cuprior while minimizing potential risks. Through its targeted action, Cuprior improves the quality of life for individuals affected by Wilson’s disease, providing a pathway to better health and well-being.
| Package | 96 tablets of 150 mg |
|---|
Be the first to review “Buy Cuprior (trientine) Online” Cancel reply
Related products
New medicines
New medicines
New medicines
New medicines
New medicines







Reviews
There are no reviews yet.