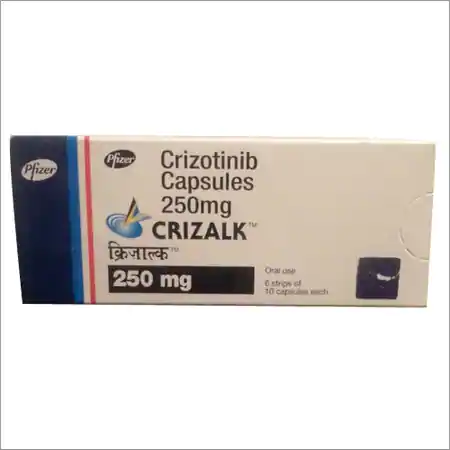
Buy Crizalk : Crizotinib 250 Mg Capsule 60’S Online
$1,068.72
Brand Name : Crizalk
Composition : Crizotinib
Manufactured by : Pfizer Pharmaceuticals, Inc.
Strength : 250 mg
Form : Capsules
Packing : Pack of 60 Capsules
Prescription Required *
Crizalk, also known as Crizotinib 250 Mg Capsule 60’S, is a medication used in the treatment of certain types of lung cancer. It is a kinase inhibitor that works by blocking the action of enzymes that promote the growth and spread of cancer cells.
Crizalk was approved by the FDA in August 2011, and since then, it has become a popular treatment option for patients with advanced non-small cell lung cancer (NSCLC) that has specific genetic mutations. These mutations involve a fusion of the ALK (anaplastic lymphoma kinase) gene or the ROS1 (c-ros oncogene 1) gene, which are present in a small percentage of NSCLC patients.
The drug is taken orally, in the form of pills or capsules. It works by inhibiting the protein kinase activity of the ALK and ROS1 genes, preventing the growth and spread of cancer cells. By blocking the activity of these genes, Crizalk has been able to significantly improve survival rates and quality of life for patients with NSCLC.
Crizalk has demonstrated impressive results in numerous clinical trials. In a Phase III clinical trial, patients who were treated with Crizalk showed a 55% overall response rate and a median progression-free survival (PFS) of 7.7 months. In another study, patients who had previously received chemotherapy and were treated with Crizalk showed a 60% overall response rate and a median PFS of 8.9 months.
One of the most significant advantages of Crizalk is the manageable side-effect profile. Most patients experience mild side effects, including fatigue, nausea, and diarrhea. However, these side effects typically resolve within a few weeks of treatment. Additionally, unlike traditional chemotherapy treatments, Crizalk does not cause severe side effects like hair loss and severe nausea.
Despite the success of Crizalk, there are some limitations to its use. The drug is only effective in patients with specific genetic mutations, which can limit its use in other types of cancer. Additionally, Crizalk may interact with other medications, and patients with liver or kidney problems may not be eligible for treatment with the drug.
In conclusion, Crizalk has been a revolutionary treatment option for patients with NSCLC who have specific genetic mutations. Its efficacy, tolerability, and manageable side effects have made it a preferred treatment option for many cancer patients. The drug has a promising future as researchers continue to explore its potential uses in other types of cancer and combination therapies.
Be the first to review “Buy Crizalk : Crizotinib 250 Mg Capsule 60’S Online” Cancel reply
Related products
Anti Cancer
Anti Cancer
Anti Cancer
Anti Cancer









Reviews
There are no reviews yet.