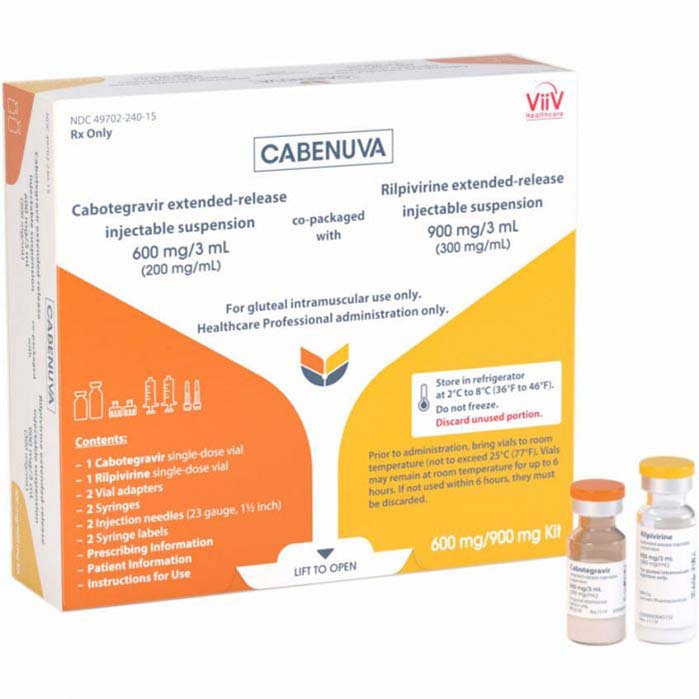
Buy Cabenuva (cabotegravir + rilpivirine) Online For Sale
From $3,635.00
DISEASE INDICATIONS: HIV/AIDS
MANUFACTURER: ViiV Healthcare
MEDICINE APPROVED BY:
Food and Drug Administration (FDA)
Health Canada
Therapeutic Goods Administration (TGA)
Cabenuva is a complete and injectable regimen for the treatment of HIV-1 in adults. It utilizes cabotegravir and rilpivirine, two drugs that reduce the viral load in patients, preventing the spread of the virus.
Cabenuva (cabotegravir + rilpivirine) is an injectable, complete regimen for the treatment of HIV-1 in adults. It is an innovative HIV treatment option that is administered once a month, which can also be used for HIV-1 patients who have previously been treated with antiretroviral therapy but have been stable. The two-drug combination, cabotegravir and rilpivirine, can suppress the viral load within the individual, preventing HIV-1 from advancing and reaching life-threatening stages.
The HIV medication has been approved by the US Food and Drug Administration (FDA) as a long-acting injectable formulation, providing convenience and discretion to patients compared to frequently oral intake of medication. Cabenuva’s once-a-month injection format eliminates the need for HIV patients to be exhausted by daily oral pills and reduces the possibility of forgetting to take their medication while enhancing efficiency.
Cabotegravir and rilpivirine are both HIV-1 drugs that work to reduce the viral load and prevent the spread of the virus. Still, unlike other oral medications, they are administered via intramuscular injection. Once injected, the drugs slowly release into the bloodstream over an extended period, maintaining therapeutic concentrations in the body for up to four weeks.
The medication comes as a two-injection therapy, where patients receive the cabotegravir injection first, followed by the rilpivirine injection a week after. In clinical trials, Cabenuva proved effective in reducing patients’ viral loads to undetectable levels, providing a viable alternative to oral HIV medication therapy.
The safety of Cabenuva has been demonstrated in clinical trials with treatment-emergent adverse effects of low frequency and mild intensity. Development of certain medicinal products can cause side effects, and Cabenuva is no exception. Common side effects of Cabenuva include pain, redness, and swelling at the injection site, fever, fatigue, muscle aches, headaches, and gastrointestinal symptoms.
In summary, Cabenuva (cabotegravir + rilpivirine) is a long-acting, complete injectable regimen for the treatment of HIV-1 in adults. It works by reducing the viral load in patients, preventing the progression of HIV-1 and potentially offering a more viable alternative to oral medication. Cabenuva is administered once a month, with cabotegravir and rilpivirine both released into the bloodstream over an extended period. The treatment is designed to improve patient convenience and discreetness while maintaining therapeutic concentration in the body. While immunocompromised people with advanced HIV should not receive Cabenuva, the safety of the regimen has been demonstrated in clinical trials, with occasional mild side effects noted.
| Package | 1 vial of cabotegravir 400 mg and one vial of rilpivirine 600 mg, 1 vial of cabotegravir 600 mg and one vial of rilpivirine 900 mg |
|---|
2 reviews for Buy Cabenuva (cabotegravir + rilpivirine) Online For Sale
Add a review Cancel reply
Related products
Infectiology
Infectiology
Dermatology
Infectiology
Infectiology
Infectiology






Dr. Alicia Nguyen, Infectious Disease Specialist –
Cabenuva has truly changed the landscape of HIV care. For eligible patients, the monthly long-acting injectable simplifies adherence and reduces stigma associated with daily pills. In my clinical experience, patients report high satisfaction and sustained viral suppression. It’s especially beneficial for those with adherence challenges or lifestyle factors that make daily dosing difficult. The safety and efficacy data are strong, and the transition from oral lead-in to injectable has been smooth for most. Cabenuva offers an exciting and empowering alternative in HIV treatment.
Jason M. –
Switching to Cabenuva has been the best decision I’ve made since starting HIV treatment. After years of taking daily oral medication, the once-monthly injection gives me so much freedom and peace of mind. I don’t have to worry about missed doses, pill fatigue, or carrying meds while traveling. The injections are quick and well-tolerated—just mild soreness for a day or two. My viral load remains undetectable, and my quality of life has drastically improved. It feels like I finally have control again.