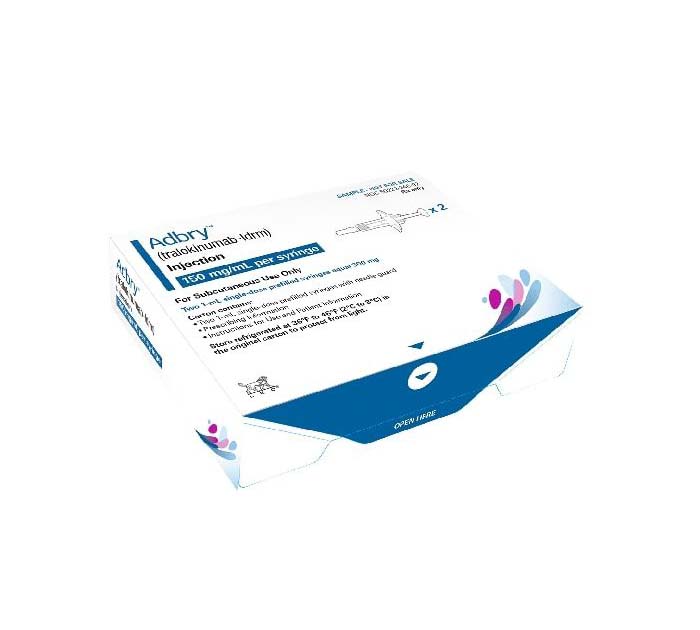
Buy Adbry (tralokinumab-ldrm) Online For Sale
$1,275.00
DISEASE INDICATIONS: Atopic Dermatitis
MANUFACTURER: Leo Pharma
USAGE: Subcutaneous
MEDICINE APPROVED BY:
Food and Drug Administration (FDA)
Adbry is a medication that helps treat moderate-to-severe eczema. It is an injectable drug that blocks the activity of IL-13, which is a cytokine responsible for inflammation in eczema. A healthcare provider or trained patient/caregiver should administer the recommended dosage of 300 mg subcutaneously. Clinical studies have shown improvement in the symptoms of eczema.
Adbry (tralokinumab-ldrm) is a prescription medication that is used to treat moderate-to-severe eczema in patients aged 18 years and older. It is an injectable drug that can be self-administered subcutaneously.
Drug Class:
Adbry belongs to a class of drugs called interleukin 13 (IL-13) inhibitors.
Indications:
Adbry is indicated for the treatment of moderate-to-severe eczema in adult patients who are candidates for systemic therapy.
Mechanism of Action:
Eczema is a chronic inflammatory skin condition that is believed to be caused by various factors, including immune system dysfunction. Adbry works by blocking the activity of IL-13, which is a cytokine that plays an important role in the inflammation associated with eczema.
Dosage and Administration:
The recommended dosage of Adbry is 300 mg, administered subcutaneously every 2 weeks for the first three doses, followed by every 4 weeks thereafter. The medication should be administered by a healthcare provider or by a trained patient/caregiver.
Effectiveness:
Clinical studies have shown that treatment with Adbry can significantly improve the signs and symptoms of eczema, including itchiness, skin lesions, and overall disease severity.
Side Effects:
The most common side effects of Adbry include injection site reactions, such as pain, redness, and swelling. Other possible adverse effects include upper respiratory tract infections, headache, and nausea.
Contraindications:
Adbry is contraindicated in patients with a known hypersensitivity to tralokinumab or any of its components. It should also not be used in patients with active tuberculosis, as it can increase the risk of reactivation of the disease.
| Package | 4 pre-filled syringe x 150 mg |
|---|
3 reviews for Buy Adbry (tralokinumab-ldrm) Online For Sale
Add a review Cancel reply
Related products
Dermatology
Dermatology
Dermatology
Dermatology
Dermatology
Dermatology







Erica T. –
Adbry helped reduce the itching and inflammation that used to keep me up all night. I’m amazed at how much better my skin looks and feels. It’s not just about the appearance—it’s the comfort, the sleep, the emotional relief. If you’re battling eczema and haven’t found the right option, I highly recommend giving Adbry a try.
Michael R. –
I started Adbry after failing multiple topical and systemic treatments. Within weeks, the redness and oozing patches began to heal. The injection is easy to administer at home, and I haven’t experienced major side effects. I no longer dread getting dressed in the morning—my skin feels almost normal again.
Jasmine L. –
I’ve had moderate-to-severe atopic dermatitis since childhood, and Adbry is the first treatment that truly made a difference. The inflammation, cracking, and constant itching have drastically improved. My skin feels stronger and more resilient. It’s been months since I had a bad flare. Adbry gave me back my comfort and confidence!