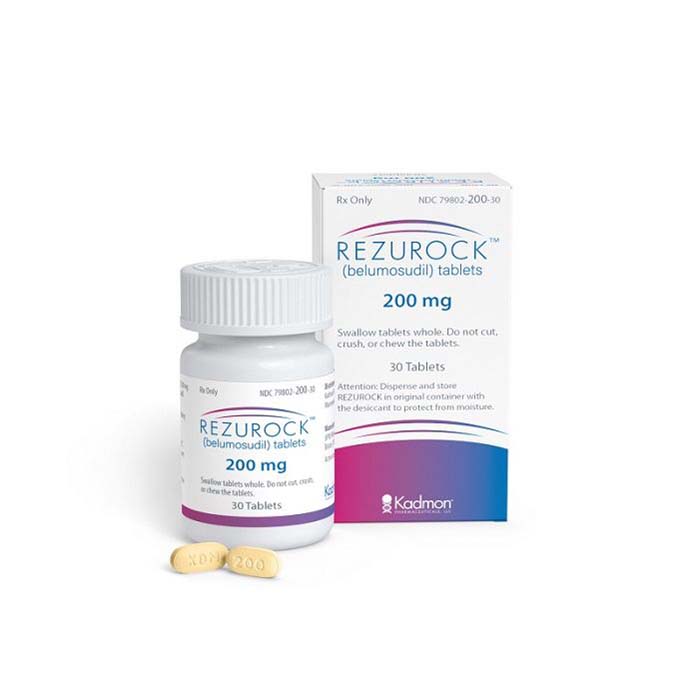
Buy Rezurock (belumosudil) Online
$177,748.00
Belumosudil is a new medication approved by the FDA for treating cGVHD in patients who failed previous therapies. By inhibiting the ROCK2 enzyme, belumosudil reduces immune cell activation to decrease cytokines and chemokines that drive inflammation, fibrosis, and tissue damage. Clinical trials on patients with cGVHD demonstrated its efficacy with residual side effects such as hypertension, diarrhea, and fatigue. The medication is administered orally twice a day and requires further testing to determine its efficacy in other immune-mediated conditions.
Rezurock (belumosudil) is a medication that was recently approved by the US Food and Drug Administration (FDA) for the treatment of chronic graft-versus-host disease (cGVHD) in patients who have failed previous therapy.
cGVHD is a common complication that can occur after allogeneic hematopoietic stem cell transplantation (HSCT), a procedure where a patient’s damaged or destroyed bone marrow is replaced by healthy stem cells from a donor source. cGVHD occurs when the transplanted immune cells recognize the recipient’s tissues as foreign and attack them, resulting in abnormal immune activation and tissue damage in multiple organs, including the skin, liver, gastrointestinal tract, lungs, and joints.
Treatment for cGVHD typically involves immunosuppressive therapy to dampen the immune system and suppress inflammation. However, until recently, limited treatment options were available for patients with cGVHD who failed first-line therapies.
Belumosudil is a first-in-class inhibitor of the ROCK2 enzyme, which plays a role in the pathogenesis of cGVHD by reducing immune cell activation. By inhibiting ROCK2, belumosudil leads to reduced production of cytokines and chemokines that drive inflammation, fibrosis, and tissue damage in cGVHD.
Clinical trials have shown that over 75% of patients with cGVHD who had previously failed at least two prior lines of systemic therapies achieved an objective response with belumosudil, as per the 5-point mGIcS score.
Belumosudil is administered orally as a tablet twice a day, and its efficacy and safety have been evaluated in two Phase III clinical trials – the ROCKstar and ROCKstar Extension trials. The studies enrolled patients with the moderate to severe forms of the disease. They received the medication twice a day, and demonstrated significant improvement in the symptoms and quality of life of patients.
The most common side effects reported were hypertension, diarrhea, and fatigue, although there may be an increased risk of infections and bleeding while on this medication.
Belumosudil represents an important advance in the treatment of cGVHD, offering a new therapeutic option for patients who have failed previous therapies. Further studies are required to determine its efficacy in a broader range of patients with cGVHD as well as its potential use in other immune-mediated conditions.
In conclusion, Rezurock (belumosudil) is a medication that was recently approved by the FDA for the treatment of cGVHD in patients who have failed previous therapy. It works by inhibiting the ROCK2 enzyme, which plays a role in the pathogenesis of cGVHD. Clinical trials have shown that it can help improve the symptoms of cGVHD and improve patients’ quality of life. The medication is generally well-tolerated but has certain side effects that must be monitored, including hypertension, diarrhea, and fatigue. Overall, belumosudil exemplifies the progress that is being made in the development of novel therapies for chronic autoimmune diseases.
| Package | 30 tablets of 200 mg |
|---|
Be the first to review “Buy Rezurock (belumosudil) Online” Cancel reply
Related products
Inflammatory Disease
Inflammatory Disease
Inflammatory Disease
Inflammatory Disease
Inflammatory Disease
Inflammatory Disease
Inflammatory Disease
Inflammatory Disease





Reviews
There are no reviews yet.