Buy Livmarli (maralixibat) Online For Sale
DISEASE INDICATIONS:
Alagille syndrome
MANUFACTURER:
Mirum Pharmaceuticals
USAGE:
Oral
MEDICINE APPROVED BY:
European Medical Agency (EMA)
Food and Drug Administration (FDA)
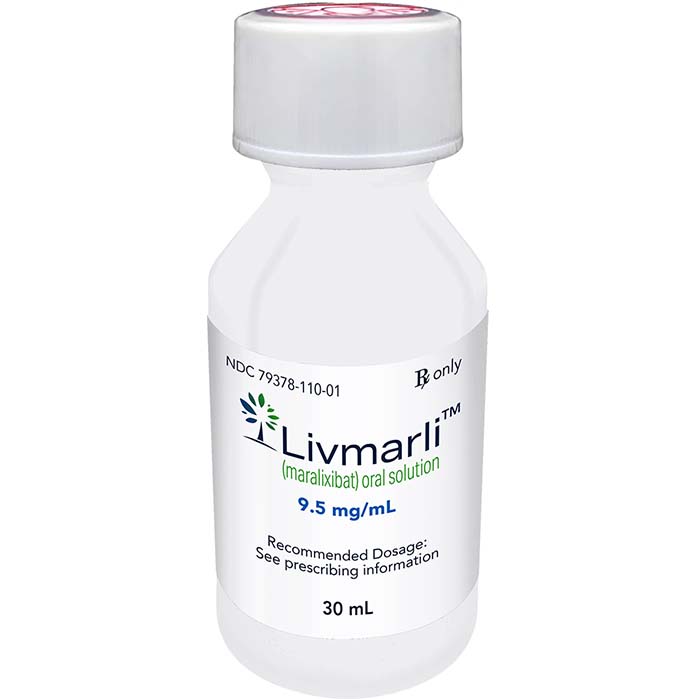
Livmarli (maralixibat) is an oral solution approved by the U.S. FDA to treat cholestatic pruritus in patients with Alagille syndrome (ALGS) who are 1 year of age or older.
Livmarli (maralixibat) is an oral solution that has been approved by the U.S. FDA to treat cholestatic pruritus in patients with Alagille syndrome (ALGS) who are 1 year of age or older. ALGS is a rare genetic disorder characterized by abnormalities in the bile ducts and liver that can lead to severe liver disease or cirrhosis. One common symptom of ALGS is persistent itching, or pruritus, which can be severe and significantly impact the quality of life of patients and their families.
Livmarli belongs to a class of drugs known as ileal bile acid transport inhibitors. It works by reducing the absorption of bile acids in the small intestine, which can lead to a reduction in the accumulation of bile acids in liver cells and a subsequent reduction in itching. Clinical studies have shown that Livmarli can significantly reduce pruritus in patients with ALGS.
The recommended dose of Livmarli for adults and children over 12 years of age is 100 mg taken orally twice daily. For children aged 1 to less than 12 years, the recommended dose is based on body weight with a maximum dose of 200 mg twice daily.
Livmarli is generally well-tolerated, with the most common side effects being diarrhea, abdominal pain, and vomiting. In clinical studies, most side effects were mild to moderate in severity and resolved over time. It is important to note that Livmarli may interact with other medications, so patients should inform their healthcare provider of all medications they are taking before starting Livmarli.
Livmarli has been shown to be an effective treatment for cholestatic pruritus in patients with ALGS, significantly improving quality of life for patients and their families. This medication provides a much-needed treatment option for a patient population with limited treatment options. As with all medications, it is important for patients to discuss potential benefits and risks with their healthcare provider and ensure that they are following all recommended guidelines for use.
In conclusion, Livmarli is an oral solution approved by the U.S. FDA to treat cholestatic pruritus in patients with Alagille syndrome who are 1 year of age or older. The medication works by reducing the absorption of bile acids in the small intestine, leading to a reduction in itching. Livmarli is generally well-tolerated and has been shown to significantly reduce pruritus for patients with ALGS, improving quality of life for patients and their families. As with all medications, it is important for patients to discuss potential benefits and risks with their healthcare provider and ensure that they are following all recommended guidelines for use.
| Package | One bottle of 9.5 mg/30 ml oral solution |
|---|
Be the first to review “Buy Livmarli (maralixibat) Online For Sale” Cancel reply
Related products
Hepatology




Reviews
There are no reviews yet.