Description
Chemical name:(Z)-2-[4-(1,2-Diphenylbut-1-enyl)phenoxy]-N,N-dimethylethanamine
Formula: C26H29NO
Anabolic activity index: not a steroid
Androgenic activity index: not a steroid
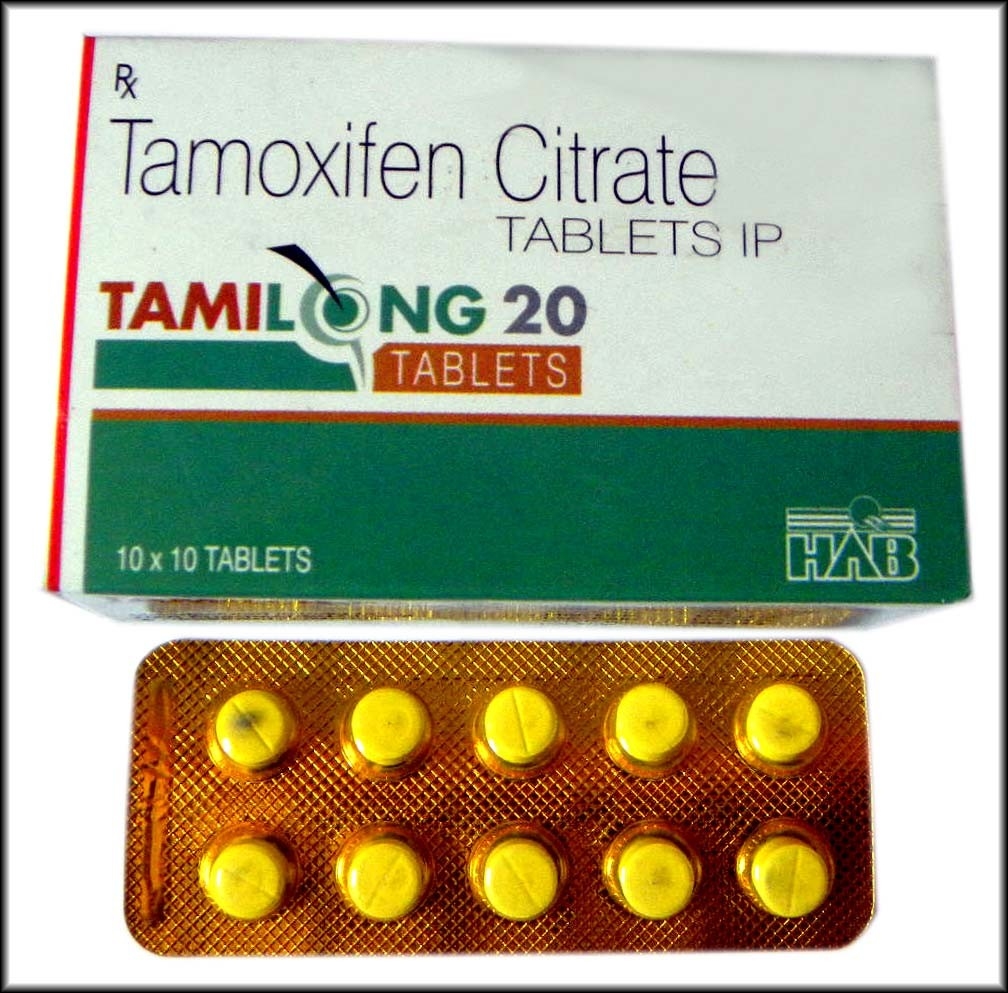
TAMILONG 20mg – HAB PHARMA
Tamilon 20 is a medication that contains the active substance tamoxifen citrate, which is classified as a selective estrogen receptor modulator (SERM). This medication is commonly used in the treatment of breast cancer, as it works by blocking the effects of estrogen in the body.
One of the key characteristics of Tamilon 20 is its relatively long half-life of 5-7 days. This means that the medication remains active in the body for a longer period of time compared to other drugs used in the treatment of breast cancer.
Tamilon 20 is typically prescribed at a dosage of 10-30 mg per day, depending on the individual patient and the severity of their condition. It is important to follow the recommended dosage instructions provided by a healthcare professional, as taking too much or too little of the medication can have negative consequences.
Unlike some other medications used in the treatment of breast cancer, Tamilon 20 is not associated with side effects such as acne, water retention, or hepatotoxicity. It also does not cause aromatization or harsh androgenic side effects.
Tamilon 20 is available in the form of 10 pills, each containing 20 mg of tamoxifen citrate. It is manufactured by HAB Pharma, a reputable pharmaceutical company that places a high emphasis on quality and safety.
In summary, Tamilon 20 is a medication that contains the active substance tamoxifen citrate, which is used in the treatment of breast cancer. It is a selective estrogen receptor modulator with a long half-life, and is available in the form of 10 pills containing 20 mg of the active substance. Tamilon 20 is known for its safety profile, lack of side effects, and effectiveness in slowing the growth of cancer cells.

Reviews
There are no reviews yet.