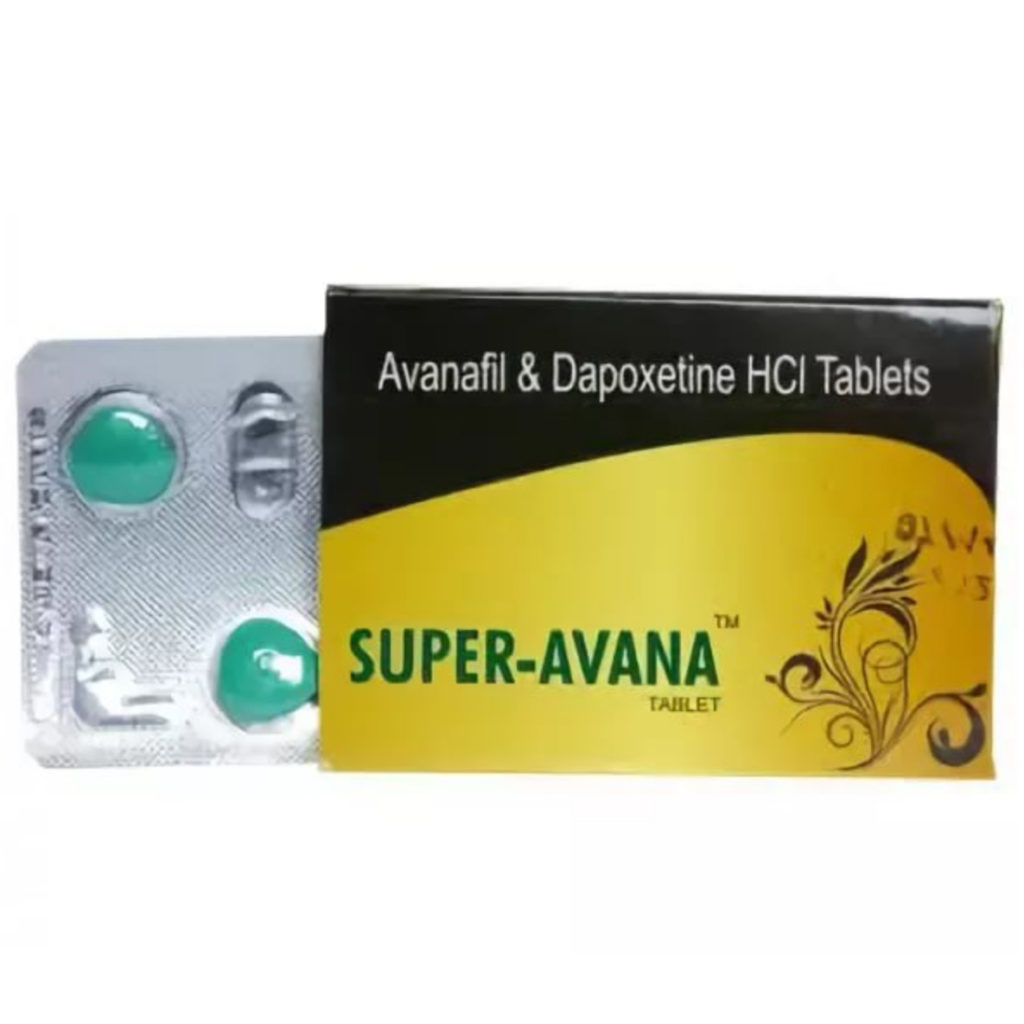
Description
Super Avana is a medication that is primarily used to treat two common sexual disorders in men, namely erectile dysfunction and premature ejaculation. It is a combination of two active substances: Avanafil, a PDE5 inhibitor that works by increasing blood flow to the penis, and Dapoxetine, an SSRI that helps to delay ejaculation.
The active half-life of Super Avana is approximately 5-10 hours, which means that the medication can remain effective in the body for this duration. Unlike other medications that only treat erectile dysfunction, Super Avana is unique in that it provides dual benefit for two common sexual dysfunctions in men.
Super Avana is classified as a combination of SSRI & PDE5 inhibitors. The recommended dosage for men is between 80-160 mg/day, and it is usually taken orally with water. In some cases, HBR or headache, back pain, and muscle pain may occur with the use of this medication.
Super Avana is generally considered to be a safe medication, and it is not associated with acne, water retention, hepatotoxicity, or aromatization. The medication is supplied in a pack of 4 pills, and each pill contains 160 mg of both active substances- Avanafil and Dapoxetine.
Super Avana is manufactured by Sunrise Remedies, one of the leading pharmaceutical companies worldwide. It is important to note that Super Avana is a prescription-only medication, and it is recommended that a man consults his healthcare provider before starting treatment with this medication.
Avanafil 100mg
Dapoxetine 60mg
Anabolic activity index: not a steroid
Androgenic activity index: not a steroid
Reviews
There are no reviews yet.